About
Clinlinc
Clinlinc company is part of the Portuguese group Noronha Sanches (NS). Founded in 2016, to start its activity in the development and manufacturing of medical devices and instruments for spinal surgery.
Clinlinc works closely with renowned neurosurgeons as well as the best international consultants, using state-of-the-art technologies to develop products aiming for an effective performance as well as a simple and comfortable technique for the surgeon.
Being a Portuguese manufacturer, Clinlinc is able to provide Portuguese surgeons with close and proactive support. Additionally, this customer engagement also gives Clinlinc the opportunity to observe and seize the market needs and requirements, which allows us to immediately identify and work on product improvements or new solutions.
Despite benefiting from the broad entrepreneurial know-how of its owning group, Clinlinc presents itself on its daily basis as a startup in evolution with a very dynamic team. These features give the company an agility and flexibility for product upgrades and new solutions that are not seen often in the industry.
Mission
and Values
Clinlinc brings those who know the most about medical devices for spinal surgery - the neurosurgeons - closer to the critical processes in product development.
By doing so, listening to their needs as well as their satisfaction with current solutions, Clinlinc seeks to develop and provide the market with constant improvements and innovations. This approach provides the surgeons with solutions that fit the requirements and specifications that they have been identifying along their careers.
Reliability|
Proximity|
Flexibility|
Transparency
Products
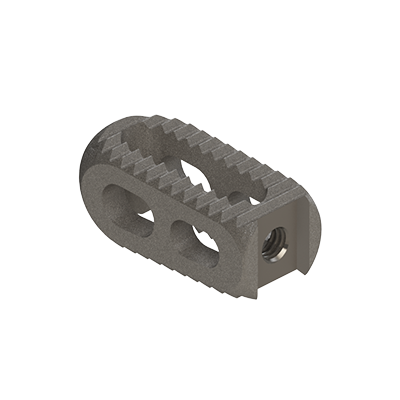
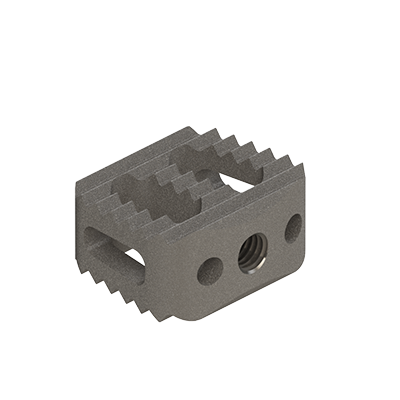
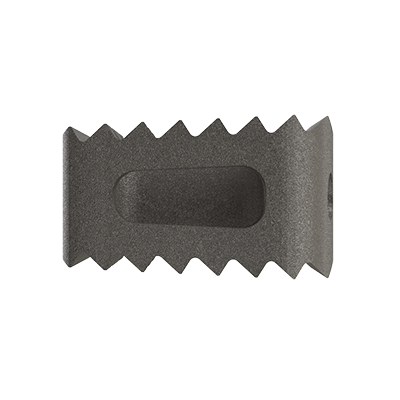
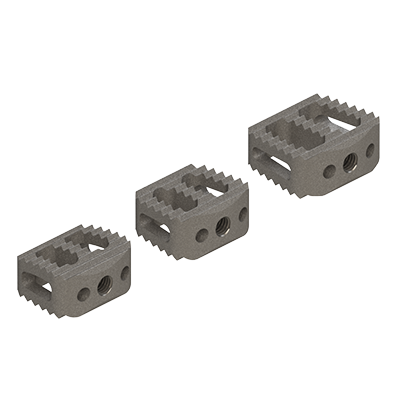
Lumbar cage
linc P
linc C
Clinlinc's titanium PLIF cage, linc P, has been developed for the stabilisation of the lumbar spine by Posterior Lumbar Interbody Fusion (PLIF).
Clinlinc's titanium cervical cage, linc C, is intended for anterior fusion of the cervical spine from levels C2 to C7.

Development
and Manufacture
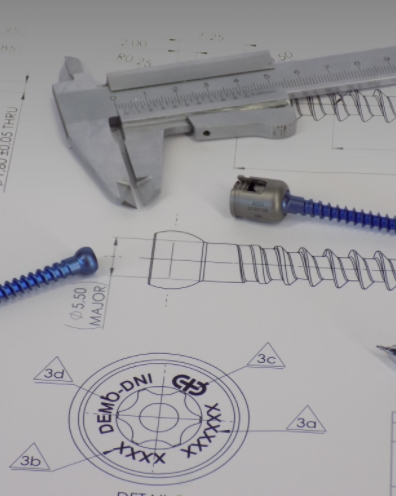
Clinlinc defines its products and ideas to start its development and manufacturing activity based on the vast knowledge and close follow-up of this industry's professionals. Once the products and their features are defined, Clinlinc resorts to the support of international consultants with many years of experience in product development, assuring that the products meet the high standards of quality and competitiveness of the industry.
Manufacture is performed by partners with decades of experience in similar medical devices for several international players.
Quality
The verification, validation and certification of Clinlinc's processes and products are conducted by accredited European entities with highly qualified experts. This assures that the products comply with the requirements of the demanding European market through a strict scrutiny.